Surface treatment
The procedure for cleaning the surfaces of the implants is rather delicate. Despite being extremely pure, the detergents utilised can leave traces on the underlying surfaces. It is possible for the few impurities present, or “the molecules of the detergent itself”, to combine with the constituents of the surface, above all in the case of reactive materials like metals. Therefore, while the cleaning tool should not be capable of chemically reacting with the device’s material, it must nevertheless be effective in eliminating any contaminants that may be present. Plasma of Argon has been found to meet these requirements.
Double acidification
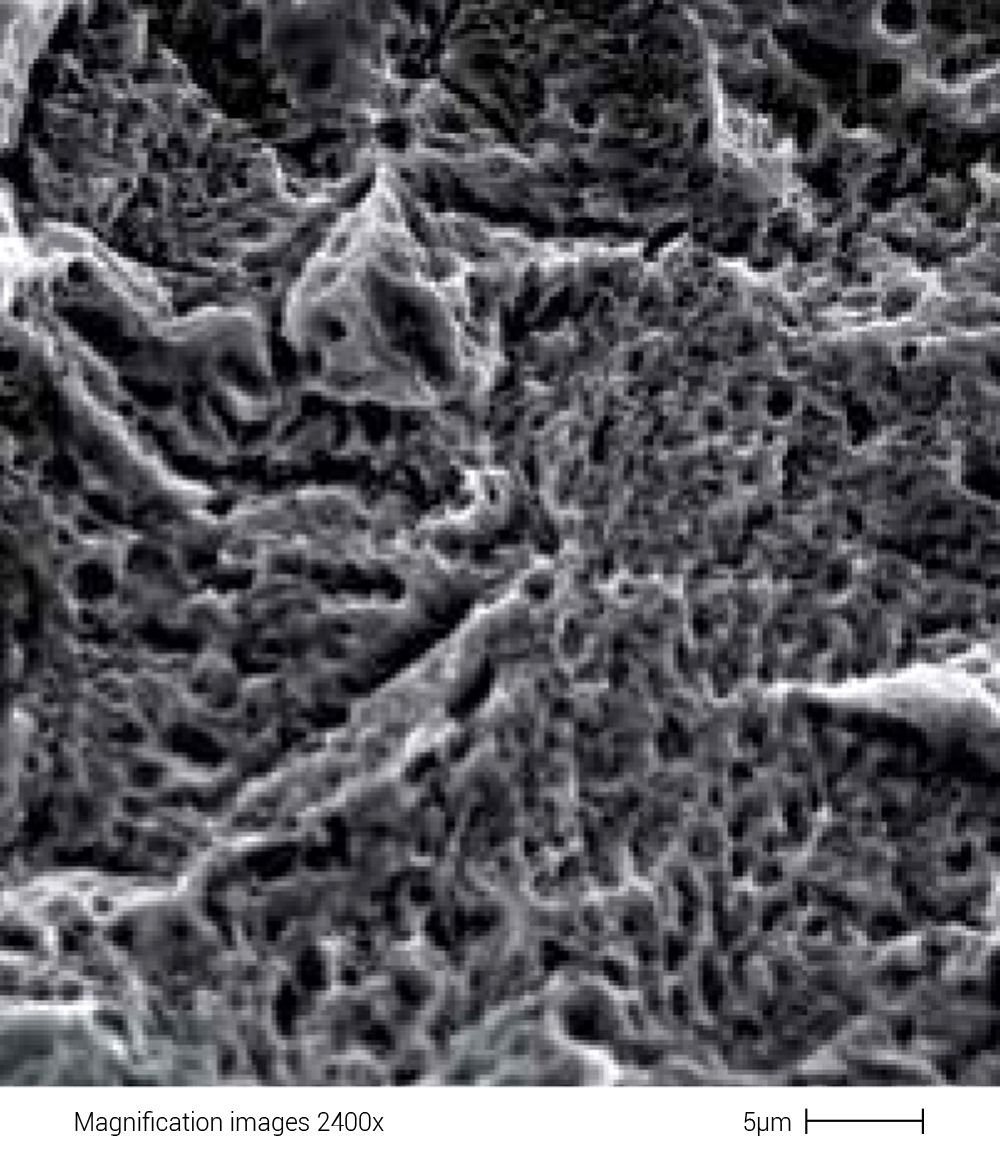
By engaging the services of international researchers with proven experience in implant surface treatment processes, Profile1® has developed a unique treatment that is capable of obtaining a surface with a controlled morphology. This subtraction treatment is designed to obtain an implant surface with a controlled microroughness of 2 microns, generating the maximum number of crestal peaks. This favours the initial cell anchorage of the osteoblasts and the subsequent integration with the bone tissue, thus decreasing the osseointegration time. It should also be pointed out that all treatments are carried out respecting strict protocols and processes through the use of technologies that ensure the uniformity of ideal surfaces with the certainty of repeatability and maintenance over time.
The plasma of argon treatment
Plasma of Argon has been identified as the ideal cleaning tool, since it does not chemically react with the device’s material, but is nevertheless extremely effective in eliminating the contaminants present on the implant’s surfaces.
In particular, the Argon gas is introduced into a reactor located in a class ISO6 clean room in order to avoid any possible environmental pollution, and is subsequently transformed into plasma. This consists of heavy gas ions, which are bombarded onto the surface of the implant, and the cleaning effect is obtained from the impact energy of its particles with any organic contaminants present. This allows for any contact with solvents to be avoided.
In order to verify the effectiveness of the process, advanced analysis techniques specifically designed for the surfaces of implant screws are utilised. In particular, an X-ray photoelectron spectroscopy (XPS or ESCA) is carried out, which is especially suitable for rough surfaces.
This type of analysis provides information about the qualitative and quantitative chemical composition of the surface material’s initial nanometres, or rather the layers that come into the most direct contact with the bone tissue..
Surface topography evaluation of Profile1® implants using the “BioActive” technique
The purpose of this job was to evaluate the surface morphology obtained following the treatment of Profile1® implants using a double acid treatment process.
Materials and methods
The surface morphology of the Profile1® implants was assessed using a scanning electron microscope (SEM). The quantitative evaluation of the roughness was performed using a roughness gauge equipped with data processing software that allows the conventional SEM image to be transformed into a three-dimensional image.